12 Which of the Following Involves a Decrease in Entropy
Click hereto get an answer to your question Which of the following process involves decrease in the entropy of system. Without an input of energy organisms would tend toward decreasing entropy.

19 7 Dg And K As Functions Of Temperature Chemistry Libretexts
During which of the following processes does the entropy of the system decrease.

. Turning carbon into diamond. This leads to a decrease in randomness. D Mixing is the entropy increasing process.
E is wrong. Helium escaping a balloon b. If a chemical reaction has a positive change in entropy Math Processing Error ΔS.
So entropy decreasing process due to decrease in disorderness of the molecules in the solid. This orderliness off a. Entropy the measure of a systems thermal.
E NaCl s Na aq Cl. Answer to All the following involve a decrease in entropy except condensation crystallization formation of a precipitate diluting a weak base. This is accompanied by an entropy decrease.
Yes this can really happen d. While there are some processes that increase entropy we also have some process that decrease entropy. Ice melts in your hand.
Which of the following involves a decrease in entropy. Freezing of liquid water into ice. Which one of the following processes involves a decrease in entropy the decomposition of NH3g to H2g and N2g the condensation of steam to liquid water the sublimation of CO2 from a solid to a gas the evaporation of ethanol the.
This local decrease is offset by an increase in entropy to the universe. The sublimation of carbon dioxide. Iron and oxygen gas react to form rust.
So remember entropy has to do with the measure off. O reactions that separate monomers. Entropy is a measure of randomness disorder of a system.
At the boiling point liquid and gas phases exist at equilibrium. Condensation involves going from the gaseous state to the liquid state. Cells require a constant input of energy to maintain their high level of organization.
The evaporation of ethanol. Conversion of ice into water. Choice B involves 1 mol gas2 mol gas and thereforeS 0.
The freezing of liquid water into ice. B Cells require a constant input of energy to maintain their high level of organization. These chemical reactions refer to the taking out of water from a.
Join Login Class 11 Chemistry Thermodynamics Spontaneity Which of the following proc. Class 12 Atoms. Which of the following process involves decrease in the entropy of system.
This means going from a less ordered state to a more ordered state. Conversion of water into steam. A Every energy transformation by a cell decreases the entropy of the universe.
Electrons being ejected from atom in a chemical reaction c. Choice A involves 3 mol gas 2 mol gas and thereforeS 0. Salt crystals dissolve in water.
C Freezing means solidification of liquid to solid. Evaporative heating of water. The disorder of the system increases.
The dissolution of NaCl in water. View the full answer. Up to 256 cash back Which of the following involves a decrease in entropy.
Every energy transformation by a cell decreases the entropy of the universe. The formation of complex molecules involves a decrease in entropy locally. A A B Freezing of water will decrease entropy as particles will move closer and forces of attraction will increase.
A the evaporation of ethanol b the condensation of steam to liquid water c the dissolution of sodium chloride in water d the sublimation of dry ice CO 2 s CO 2 g e the decomposition of NH 3 g into H 2 g and N 2 g gas. Entropy increase due to increasing randomness of gaseous molecules. Which of the following proc.
In addition for a system at equilibrium ΔG 0. Conversion of energy from one form to another is always accompanied by some gain of free energy. D Without an input of energy organisms would tend toward decreasing entropy.
H20 s -- H2O 1 O A. Air escapes from a hole in a balloon. Which of the following processes involves a decrease in entropy.
A condensation reactions B reactions that separate monomers C depolymerization reactions D hydrolysis reactions. None of these decrease the entropy of the system. C Conversion of energy from one form to another is always accompanied by some gain of free energy.
Which of the following process involves decrease in the entropy of system. Which of the following involves a decrease in entropy. Which of the following involves a decrease in entropy.
For example when two liquids are mixed to form a solution. O All choices decrease entropy. Choice C involves 1 mol gas1 mol gas and thereforeS will have a small magnitude.
A decrease in the number of moles of gas from reactants to products indicates a decrease in entropy S 0. For the following process does entropy increase decrease or remain the same. D Adsorption will lead to a decrease in the randomness of gaseous particles.
Heating up a container of water. The molecules of each liquid have. Off this system you have melting off solids sublimation freezing mixing separation on boiling.
In problem 36 12 Look at which of these involved an increase in the entropy. What is an example of a system showing a decrease in entropy a.
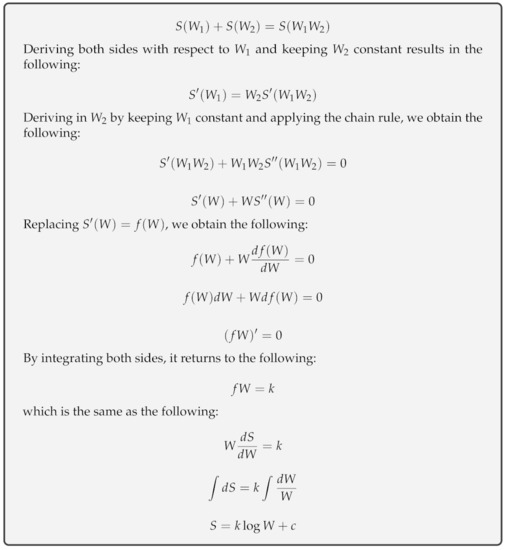
Entropy Free Full Text Entropy From Thermodynamics To Information Processing Html

Predict In Which Of The Following Entropy Increases Decreases A A Liquid Crystallizes Into A Solid B Temperature F A Crystalline Solid Is Raised From 0 K To 115 K C 2nahco3 S Nano3
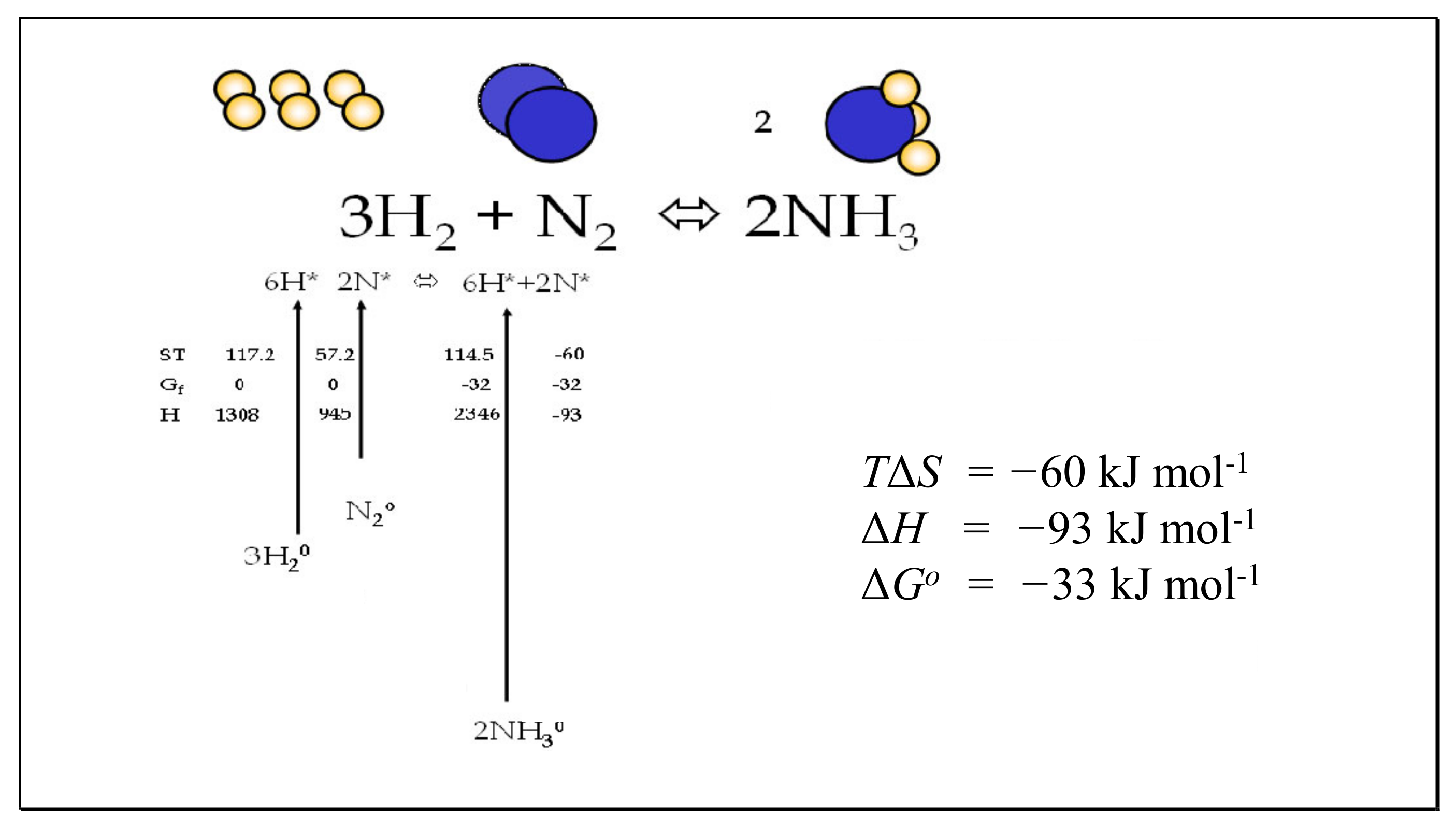
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html
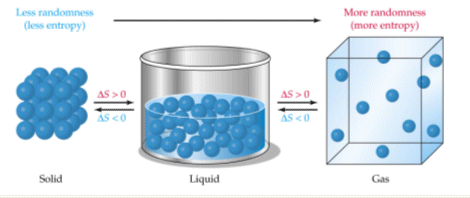
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic

506 Questions With Answers In Entropy Science Topic

Example 17 1 Predicting The Sign Of Entropy Change Ppt Video Online Download
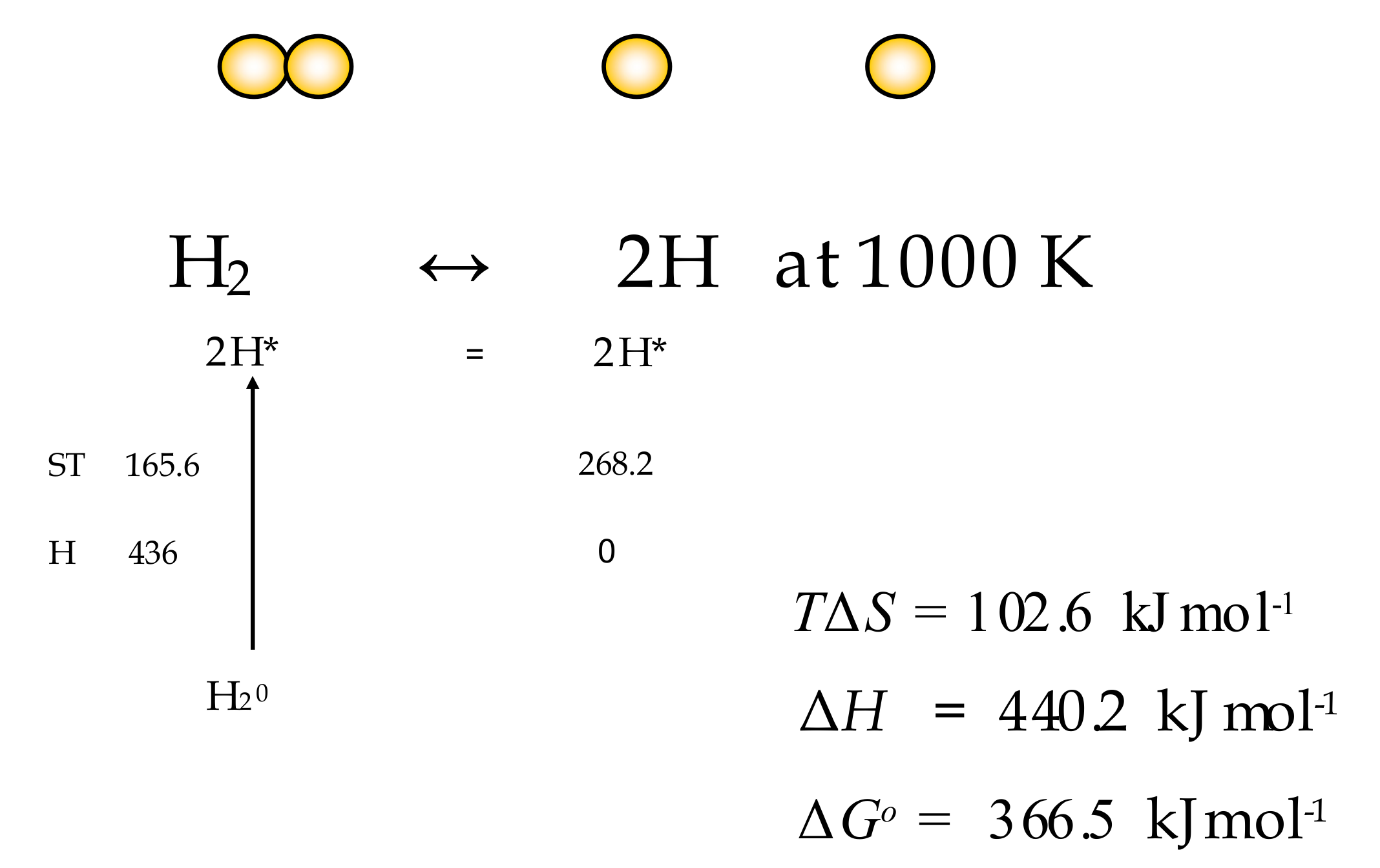
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

In Which Of The Following Processes Does The Enotropy Decrease

Which One Of The Following Demonstrates A Decrease In Entropy

Pdf Entropy Decrease In Isolated Systems Theory Fact And Tests
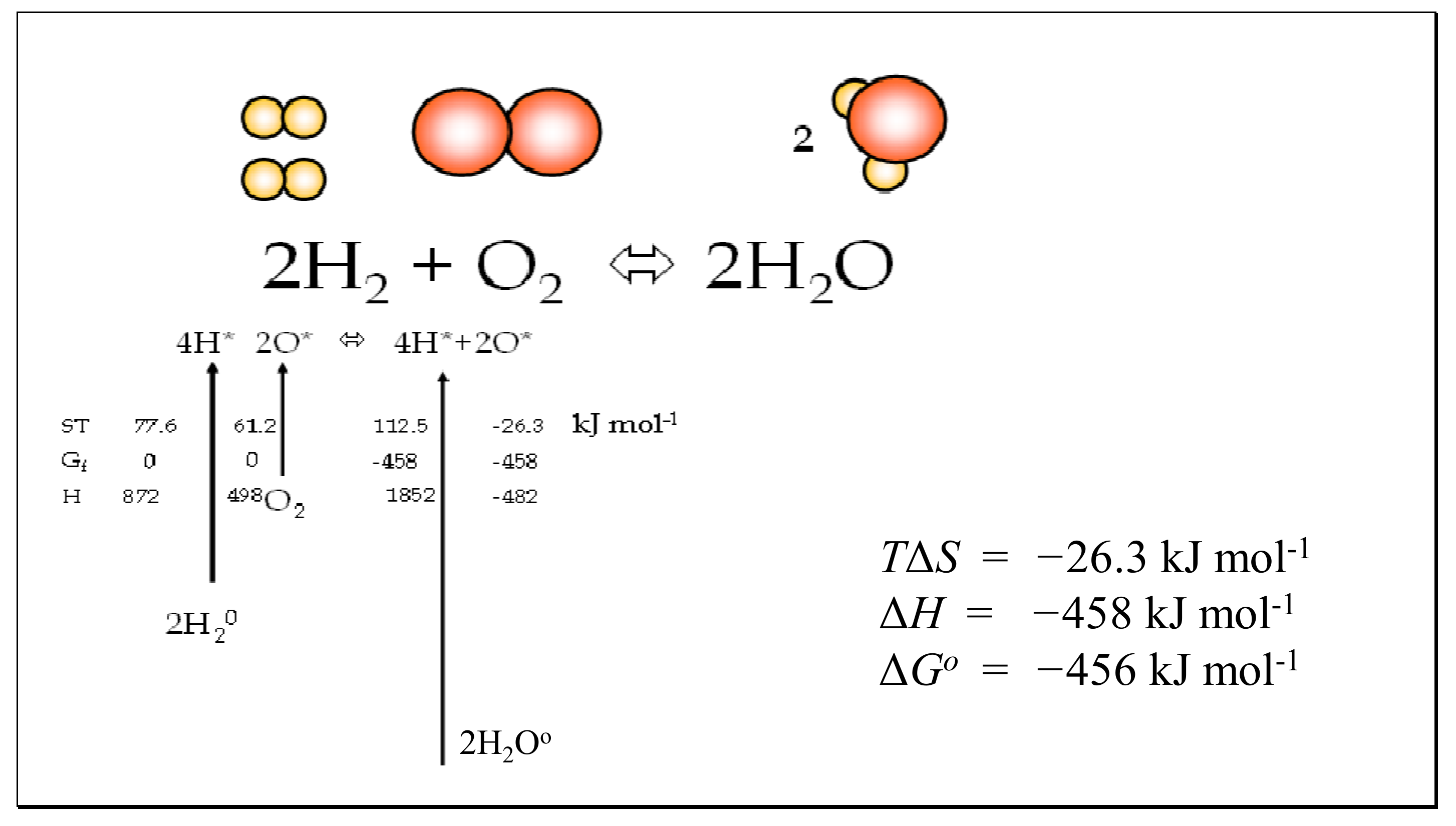
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html
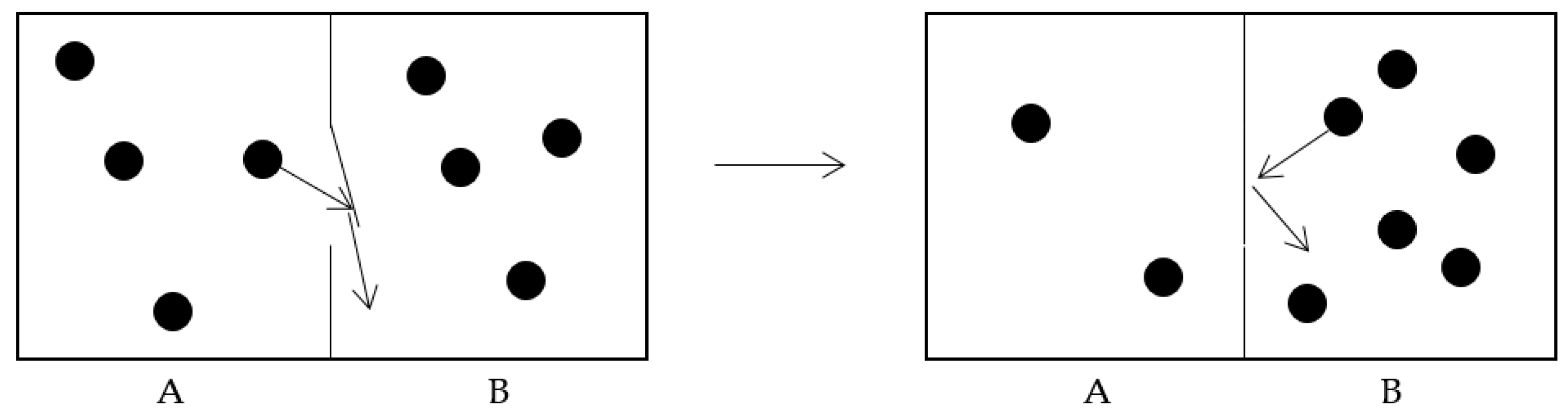
Entropy Free Full Text Maxwell S Demon A Historical Review Html
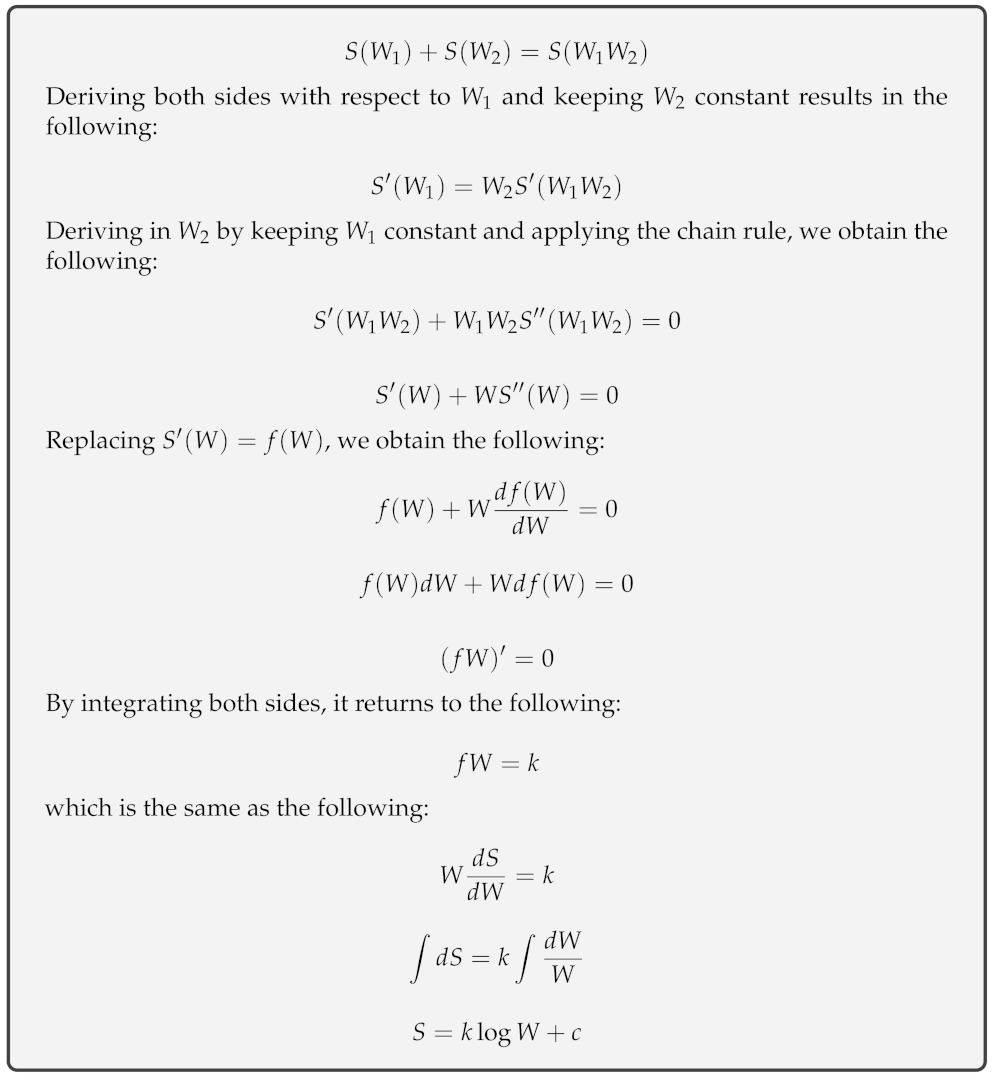
Entropy Free Full Text Entropy From Thermodynamics To Information Processing Html
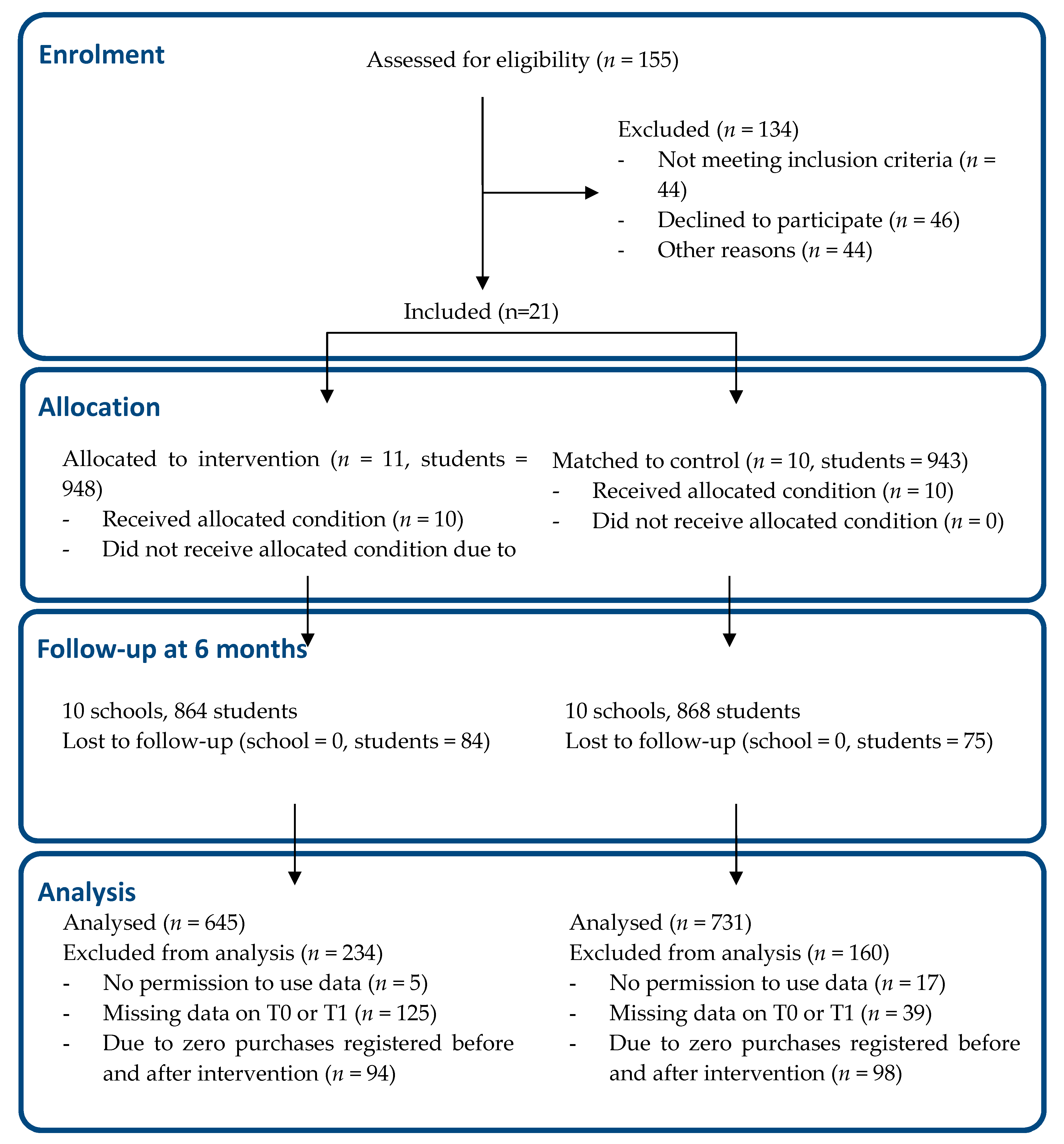
Nutrients Free Full Text The Effect Of Supportive Implementation Of Healthier Canteen Guidelines On Changes In Dutch School Canteens And Student Purchase Behaviour Html
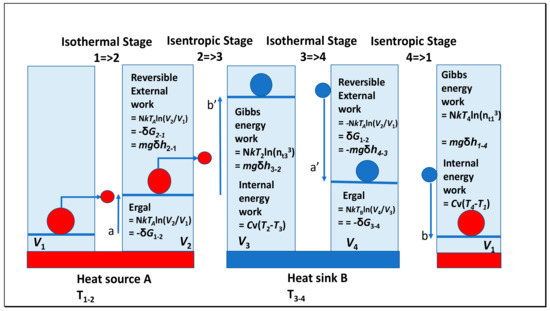
Entropy Free Full Text Partitioning Entropy With Action Mechanics Predicting Chemical Reaction Rates And Gaseous Equilibria Of Reactions Of Hydrogen From Molecular Properties Html

Which Of The Following Demonstrates A Decrease In Entropy
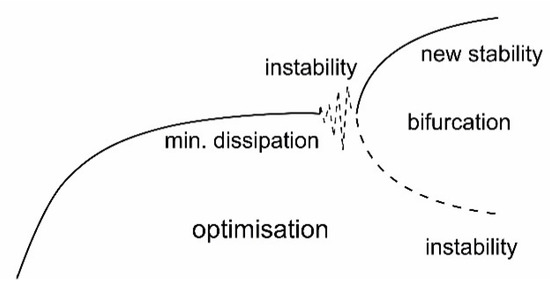
Entropy Free Full Text Thermodynamics In Ecology An Introductory Review Html

